Suitable for patients across all risk groups
The ArteraAI Prostate Test can be used for patients across all NCCN risk groups, with 10-year risk of distant metastasis (DM) and prostate cancer specific mortality (PCSM) reported and can be used to assess how aggressive the cancer is.
For lower-risk patientsFor patients with NCCN very low-, low-, or favorable intermediate-risk disease., the ArteraAI Prostate Test will also report the relative risk of adverse pathology (AP) at radical prostatectomy (RP) to help inform if active surveillance is a suitable management option.
For intermediate-risk patients, the ArteraAI Prostate Test can be used to predict if there will be a therapeutic benefit from adding ST-ADT to radiation therapy.
For higher-risk patientsFor patients with NCCN high-, or very high-risk disease., the ArteraAI Prostate Test can help inform if adding abiraterone to LT-ADT and radiation therapy may be of benefit.
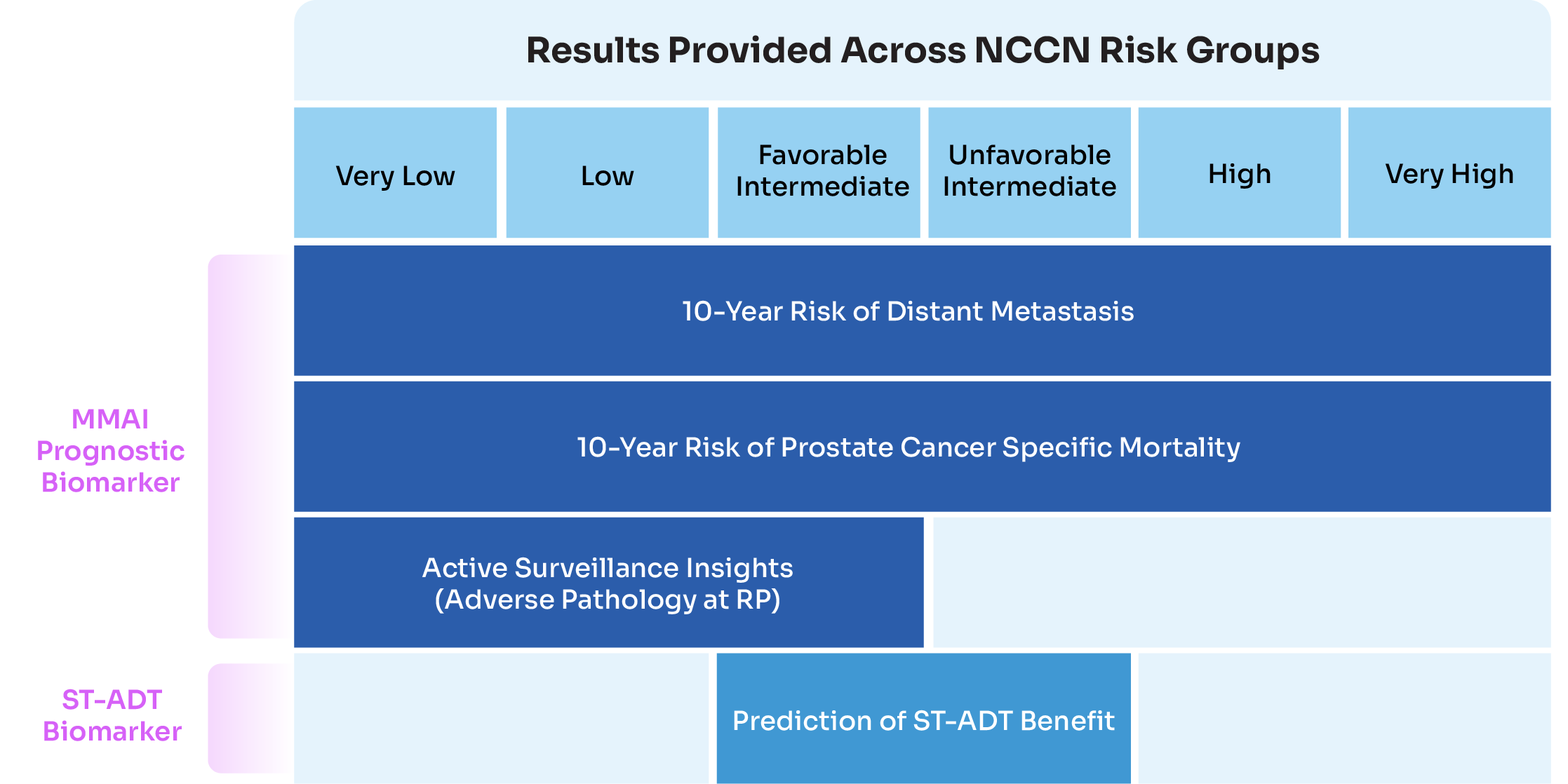
The ArteraAI Prostate Test is intended to assist clinicians with risk-based decisions in localized prostate cancer within recommended clinical treatment guidelines. This multimodal artificial intelligence (MMAI) test is recommended for patients with localized prostate cancer in the NCCN Guidelines for Prostate Cancer*Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.4.2026.© National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed December 18, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way..
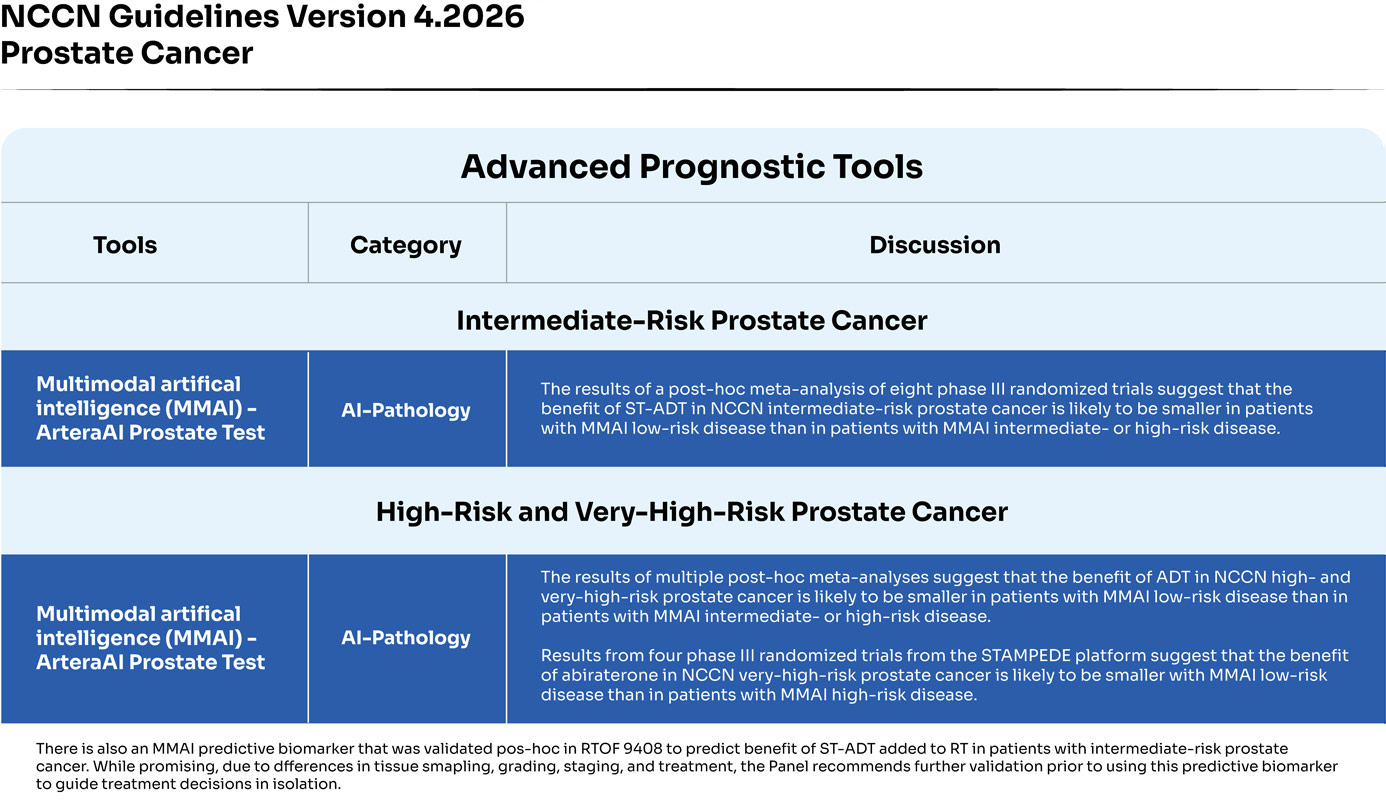
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.42026. © 2025 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available.
The science behind the ArteraAI Prostate Test
Artera’s multimodal artificial intelligence (MMAI) platform leverages a unique algorithm that assesses both the digital images from a patient’s biopsy and their clinical data. The AI combines this information to estimate long-term outcomes and predict whether a patient will benefit from hormone therapy.
The algorithm was developed using large datasets, from thousands of patients and tens of thousands of pathology slide images, and has been clinically validated using multiple Phase 3 randomized trials and in a variety of patient cohorts.
Ordering is Easy with Artera
The ArteraAI Prostate Test is a laboratory-developed testTesting is performed by ArteraAI, located at 6800 Southpoint Pkwy Suite 950, Jacksonville, Florida 32216. The ArteraAI Prostate Test was developed and its performance characteristics were determined by ArteraAI. This Laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ‘88) as qualified to perform high-complexity clinical laboratory testing. This test is used for clinical purposes and should not be regarded as investigational or for research. This test has not been cleared or approved by the U.S. Food and Drug Administration. that is now clinically available through a single CLIA-certified laboratory in Jacksonville, FL.
-
The ArteraAI Prostate Test is intended for adult males 18 years of age or older with localized prostate cancer, without clinically or pathologically defined metastases. The intended patient should also be a candidate for curative intent management (surgery, radiation therapy ± systemic therapy, or active surveillance).
-
Yes! You can visit the ArteraAI Test Report page to explore each section of the report to better understand the results.
-
The ArteraAI Prostate Test can be ordered in all 50 US states. We also have partners in Puerto Rico, United Kingdom, Australia, and Israel where versions of the ArteraAI Prostate Test are available through our partnerships. Please reach out to support@artera.ai for additional details or if you are located outside the listed geographies and are interested in placing an order.
-
After you reach out to us, a member of our Sales team will set up an account in the ArteraAI Portal to enable order submission. You and/or an ordering delegate will receive login instructions and once an order is placed in the portal, the Customer Success Team will contact the pathology lab to arrange all necessary logistics to ship the histopathology sample to our laboratory in Florida.
Alternatively, you can fill out and fax in a Test Requisition Form to place an order as well.
-
No. Ordering of the ArteraAI Prostate Test is limited to clinicians whose license and scope of practice allows them to order the test. However, we encourage patients to share information about ArteraAI with their doctor.
-
Using the ArteraAI Prostate Test, results can be shared within 1-2 days after receipt of the patient’s specimen.
-
The out-of-pocket cost for the ArteraAI Prostate Test will be based on the patient’s insurance plan. If the patient is covered by Medicare Plan B, they should have zero out-of-pocket costs for the ArteraAI Prostate Test. If the patient is covered by private insurance, financial liability will be subject to the terms of their plan taking into consideration copays, coinsurance and deductibles. Patients should contact billing@artera.ai for additional questions about their estimated out-of-pocket liability for the test.
Order the ArteraAI Prostate Test for your patient. Start your order