Developed to Aid Post-Prostatectomy Treatment Decisions
Patients with biochemical recurrence following radical prostatectomy face the decision to augment salvage radiation therapy with ADT to further decrease their risk of poor long-term outcomes. The ArteraAI Prostate Test can help provide insight into disease prognosis by estimating the risk for developing distant metastasis and prostate cancer specific mortality, based on individual patient characteristics. The test’s prognostic risk assessment also provides insights into the potential benefit of adding ST-ADT to salvage radiation therapy. With the known side effects of ADT, such as sexual dysfunction, cognitive dysfunction, and metabolic syndrome, gaining a clear understanding of the risk-benefit of adding ST-ADT to salvage-RT could increase confidence in treatment decision-making.Nguyen PL et al. Eur Urol 2015;67(5):825-836.
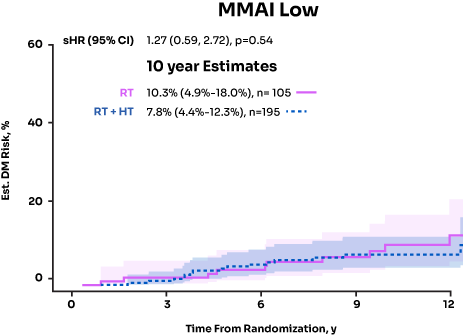
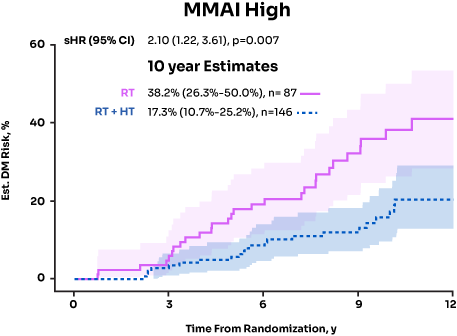
MMAI Identified Patients Who Benefitted From ADT Post Radical ProstatectomyData on File, Artera, 2024
NRG/RTOG 9601 and 0534
Ordering is Easy with Artera
The ArteraAI Prostate Test is a laboratory-developed testTesting is performed by ArteraAI, located at 6800 Southpoint Pkwy Suite 950, Jacksonville, Florida 32216. The Artera Prostate Test was developed and its performance characteristics were determined by Artera. This Laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ‘88) as qualified to perform high-complexity clinical laboratory testing. This test is used for clinical purposes and should not be regarded as investigational or for research. This test has not been cleared or approved by the U.S. Food and Drug Administration. that is now clinically available through a single CLIA-certified laboratory in Jacksonville, FL.
-
Yes! You can visit the ArteraAI Test Report page to explore each section of the report to better understand the results.
-
The out-of-pocket cost for the ArteraAI Prostate Test will be based on the patient’s insurance plan. If the patient is covered by Medicare Plan B, they should have zero out-of-pocket costs for the ArteraAI Prostate Test. If the patient is covered by private insurance, financial liability will be subject to the terms of their plan taking into consideration copays, coinsurance and deductibles. Patients should contact billing@artera.ai for additional questions about their estimated out-of-pocket liability for the test.
-
The ArteraAI Prostate Test can be ordered in all 50 US states. We also have partners in Puerto Rico, United Kingdom, Australia, and Israel where versions of the ArteraAI Prostate Test are available through our partnerships. Please reach out to support@artera.ai for additional details or if you are located outside the listed geographies and are interested in placing an order.
-
Using the ArteraAI Prostate Test, results can be shared within 1-2 days after receipt of the patient’s specimen.
-
No. Ordering of the ArteraAI Prostate Test is limited to clinicians whose license and scope of practice allows them to order the test. However, we encourage patients to share information about ArteraAI with their doctor.
-
After you reach out to us, a member of our Sales team will set up an account in the ArteraAI Portal to enable order submission. You and/or an ordering delegate will receive login instructions and once an order is placed in the portal, the Customer Success Team will contact the pathology lab to arrange all necessary logistics to ship the histopathology sample to our laboratory in Florida.
Alternatively, you can fill out and fax in a Test Requisition Form to place an order as well.
-
The ArteraAI Prostate Test (Post-RP) is intended for adult males 18 years of age or older with a biochemical recurrence following prostatectomy for localized prostate cancer and who have not yet received radiation or hormone therapy after prostatectomy.
Order the ArteraAI Prostate Test for your patient. Start your order